Vanisha Gupta, Umang Sultania, Gouri Naik, and Anamika Sharma
The uses of plants are many. There are entire industries that thrive on natural products (NPs) obtained from plants – traditional and modern pharmaceutical industries, agricultural industries, food industries, perfume industries to name a few.1,2 Extracting natural products from plants is an interesting chemical process, and we at Prayoga Institute of Education Research felt that we could use it to generate some excitement about chemistry in our students. So, we got our students to isolate volatile organic compounds (a form of NP) from plants. This experiment would also show the students how the perfume industry extracts essential ingredients to make different fragrances.
Distillation is the most common technique used to extract NPs, especially volatile organic compounds. Once the compounds were extracted, the students were to separate the compounds into different components based on polarity using Thin Layer Chromatography (TLC, a technique that separates components in non-volatile mixtures) and Ultraviolet Spectroscopy (a technique that uses ultraviolet light to determine the absorbency of a substance).
Materials and methods
General: All the reagents, chemicals, and solvents were purchased from commercial suppliers. The extracts were monitored using TLC (silica gel 60-F254 protected aluminium sheets). The Ultraviolet (UV) spectra were recorded on a Shimadzu UV-1780 UV-visible spectrophotometer using a UV probe software.
Plant material: Six different plant sources were chosen for the study: outer rim of sweet lime, mint leaves, lavender stem, allspice leaves, rosemary, and eucalyptus leaves.
Steam distillation: The setup includes a large copper container having water. The container is placed over a Bunsen burner/hot plate. The Bunsen burner/hot plate heats the water in the container, which causes the water to boil and produce steam. The container is attached to an Erlenmeyer flask (containing a flat bottom, conical body, and cylindrical neck) with a rubber tube. Steam passes through the rubber tube into the flask. The flask contains the plant material. The flask is connected to a condenser, which consists of two glass tubes: the large tube is connected to a continuous supply of water, and the smaller tube receives the volatile compounds from the plant material, which condense due to the water. The condensed substances flow through the small tube into a beaker (Figure 1).

Results and discussion: All the plant sources chosen were present at Prayoga Institute of Education Research except for sweet lime and mint (which were gift samples. The plant samples were cut into small pieces to increase the surface area, and these were then subjected to steam distillation (Figure 2). The first experiment was conducted on sweet lime. The initial weight of the sample was 129 g, which upon steam distillation afforded 60 mL extract from three fractions. Distillation was carried out for a duration of one hour.

It was found that at each consecutive fraction, the intensity of the smell increased. After three fractions, the smell decreased and the distillation was stopped. The sample collected from each fraction was mixed with fragrance (from the volatile natural products) and sealed in a vial. There was a loss of fragrance after the sample was left on a bench at room temperature for 14 days. The next sample was mint leaves. The leaves were finely chopped (initial weight of 90 g) and a similar procedure was carried out. A total of 90 mL of extract was collected from three fractions in a duration of one hour. The intensity of the smell increased with each subsequent fraction. A small amount was allowed for slow evaporation to obtain menthol crystals. But due to the low sample size, only white precipitate was obtained in very small amounts. The sample remained stable on the bench even after 14 days. Similarly, lavender stem was cut into small pieces (<1 cm in length). The initial weight was 87 g. A total of 310 mL was collected as extract in several fractions over a span of four hours of steam distillation. During the first hour, there was a light smell of lavender oil along with other volatiles from the stem. However, after 30 minutes of continuous steam distillation, the pure smell of lavender oil was obtained and the fractions were collected from that point. In the subsequent fractions, an emulsion of oil in water was obtained, which upon settlement allowed the oil to float on water (Figure 3). The distillation continued until the smell of lavender diminished from the fractions.

Fresh leaves from rosemary were cut and 85 g of the plant material was subjected to steam distillation. The initial fractions collected had a mixed smell due to the presence of other organic volatiles. After two fractions, the smell was quite intense and several fractions (290 mL) were collected until the smell diminished. The distillation was carried out for four hours. The emulsion of oil in water was quite significant as in the above case. Allspice leaves were also treated in a similar way. About 96 g of fresh cut leaves of allspice upon steam distillation for three hours afforded several fractions (total volume collected 65 mL). The intensity of the fragrance was quite high from the first fraction itself. The concentration of the extracted oil was high enough that the oil droplets were floating on water (unlike the formation of emulsion).
In the case of eucalyptus, leaves were cut into small pieces and two experiments were designed. In the first case, 98 g of plant material was steam distilled for four hours. A total of 370 mL (as several fractions) was collected. The observations were similar to those of the allspice plant. The oil droplets floating over water were quite evident, indicating the success of the process. In the second case, the same amount of freshly cut leaves and n-hexane were kept in a conical flask in the dark for seven days. The idea behind the experiment was to compare the two processes, one with heating and the other, extraction with an organic solvent.

All the plant extracts were further extracted into ethyl acetate (Figure 4). The two layers of ethyl acetate and water were then separated and evaluated further. All the fractions obtained from all plant sources were evaluated using TLC and UV spectroscopy. Several mobile phases were developed for TLC visualization at 254 nm with a proper retardation factor (Rf). Figure 5 depicts the TLCs done in different solvents (with different compositions to modify the polarity of the mobile phase).
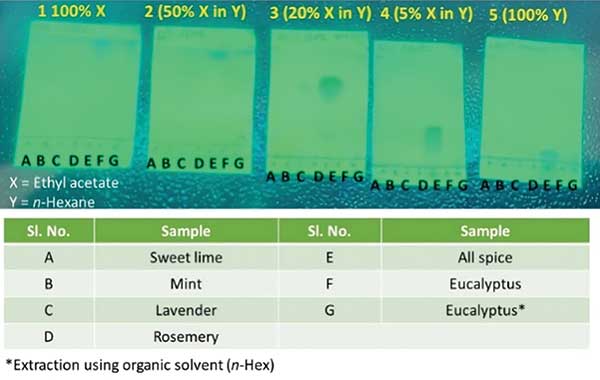
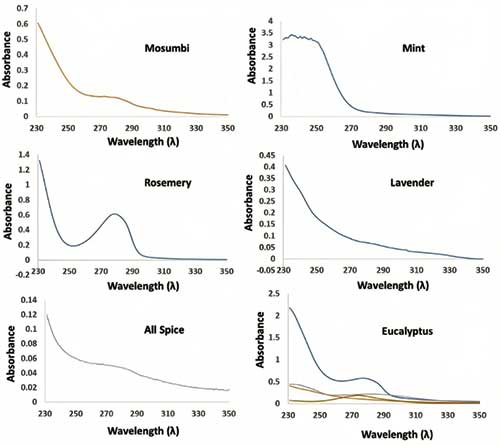
The TLCs show that in sample A-D very faint spots were visible, and for E-G, the spots were quite evident. The polarity was decreased from plate 1 to 5 (from 100% ethyl acetate to 100% n-Hexane). UV spectroscopy was also performed on the samples (Figure 6). The λmax lies in the range of 270-310 nm, which indicates the presence of aromatic heterocycles and conjugated system. In the case of eucalyptus, the UV for extraction using organic solvent (blue line) was found to have more absorbance compared to steam distillation.
Conclusion
NPs represent a large and diverse group of organic compounds produced from living organisms. Plants produce a vast and diverse variety of organic compounds, the majority of which do not emerge to participate clearly in the growth and development of the plants. Thus, newer approaches need to be evaluated to extract these volatiles to maximize application.
Acknowledgement: We thank Prayoga Institute of Education Research for providing the laboratory to carry out the project. We thank Dr. K. S. Nagabhushana for fruitful discussions regarding the implementation of the project.
References
- Sarker, S. D.; Nahar, L., Nat. Prod. Isolation 2012, 1-25.
- Katz, L.; Baltz, R. H., J. Ind. Microbiol. Biotechnol. 2016, 43(2-3), 155-176.
Vanisha Gupta is a student at The Samhita Academy, Lakshmipura, Bangalore.
Umang Sultania is a student at PSBB Learning Leadership Academy, Lakshmipura, Bangalore.
Gouri Naik is a student at Sri Kumarans Children’s Home, Mallasandra, Bangalore.
Anamika Sharma works with the Prayoga Institute of Education Research, Department of Chemistry, Bangalore. The authors can be reached at anamika.aug14@gmail.com.
