Savita Ladage and Hanza George
Chemistry is one of the core science domains taught in school. In the simplest terms, chemistry deals with materials and their transformations. Thus, it is closely associated with our lives and is important for us to understand this association. However, these connections are not always apparent through the content taught in schools. In fact, chemistry seems very abstract when it is introduced in the syllabus. The abstract nature poses several challenges to learners and teachers. While struggling to understand the concepts, learners often end up having misconceptions. This article aims to help teachers understand why chemistry poses learning challenges and to acquaint them with some of the standard resources available in the public domain.
Learners’ misconceptions in chemistry is an established area in the field of Chemistry Education Research (CER). It is important to not associate negative connotations with the word misconceptions. The suffix ‘mis‘ indicates the variation in the learners’ conceptions from the current scientific understanding of chemistry concepts. We also would like to emphasize that any individual, not just school students, can have misconceptions.
There are several reasons for developing misconceptions. For example, in everyday life, we observe that combustion leads to a massive reduction in volume or mass of materials. Thus, it is a counter-intuitive experience for learners to understand that substances can gain mass after combustion. Sometimes language can be a barrier, e.g., ‘salt’ in chemistry is a technical term (a substance produced by the reaction of an acid with a base), but often it is perceived by learners as referring to sodium chloride (namak in the Indian context). Another crucial factor responsible for misconceptions in chemistry is related to wrong instructional practices. It is, thus, important to first understand the characteristics of chemistry as a subject.
The late Prof. Alex Johnstone, a renowned chemistry education research expert from the University of Glasgow, has described three major components of modern chemistry, which is the chemistry taught in schools today (see Figure 1). These major components are – a) Macro, b) Sub-micro and c) Representational components.
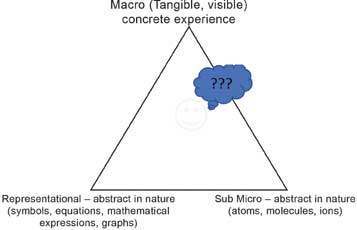
Figure 1: Three components of the modern chemistry-chemistry triangle (by Alex Johnstone).
The macro component of chemistry (that is, descriptive chemistry) represents the concrete experiences that one can gather about the material and their transformations through sense organs, e.g., texture or appearance of materials, changes during chemical reactions such as a change in colour, precipitation, the formation of gases/effervescence and change in temperature during the course of a reaction. The sub-micro component represents concepts such as the particulate model of matter, subatomic particles, etc. There are no direct experiences related to this abstract and theoretical sub-micro component. The explanations in chemistry are in terms of the sub-micro component. The third representational component (the language of chemistry), includes equations, formulae, mathematical/graphical representations, etc., which is equally abstract in nature.
When serious engagement with chemistry begins at school, learners have some experiences relating to materials, that is the learners are located at the macro end of the triangle. Soon, they are pushed towards the middle of the triangle with the introduction of the other two components (over grade 7-9). In fact, the learners are not even presented with enough activities for the exploration of materials, understanding their properties, patterns in these properties and inter-linkages between properties and uses. In fact, learners are expected to comprehend all the three components of chemistry thus placing a high cognitive load on them without sufficient support. It is not surprising if learners develop misconceptions in such a scenario.
The teaching of abstract components is equally challenging for teachers who struggle to find ways to help learners comprehend these aspects. Awareness about misconceptions helps teachers reflect on their teaching strategies and the analogies, drawings, simulations, etc., to be used while teaching concepts in chemistry.
Let us now look at a few useful resources related to misconceptions. These include worksheets that can be used in the classrooms. All these resources are from the website of the Royal Society of Chemistry, UK and are in the public domain. Figure 2 tells you how to access these resources.
| Figure 2: How to get access to the worksheets? You need to register yourself with edu.rsc.org, free of cost. You can use the link given below to access their page. Link: https://edu.rsc.org/resources Then on the search bar, type the keywords – ‘chemical misconceptions’ You can go through various available resources. Download and use the ones that are most relevant to your learners’ needs. |
A good number of these worksheets are from the book titled Chemical misconceptions – prevention, diagnosis and cure – Volume 2 written by an established chemistry education researcher, Prof. Keith S Taber, currently Professor of Science Education at the University of Cambridge. Information about the book is also available on the RSC website. The worksheets present target age group, rationale, instruction for usage and answer keys for teachers.
Even though the materials are linked to the UK chemistry syllabus, they are relevant for chemistry teachers in India. Some of the resources present a detailed commentary about a topic that has pedagogical significance. We believe that some of the worksheets can be easily translated (with permission of RSC) into worksheets in other languages for use in schools where the medium of instruction is not English. We hope motivated teachers use these sheets for their classrooms and benefit from the same.
We also would like to point out that misconceptions will not be rectified immediately. It is important to present opportunities to elicit and have discussions about misconceptions to sensitize learners about their thinking. Awareness about misconceptions provides useful insights about some of the tools that can be used in tuning the teaching for deeper learning of chemistry.
AFL: Introducing particle models
*https://www.rsc.org/education/teachers/resources/aflchem/resources/19/index.htm
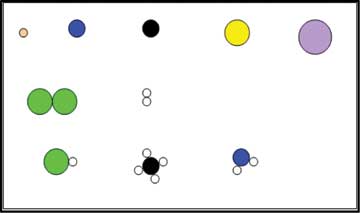
These are a set of cards which represent matter using different types of particles (see Figure 3 – an actual figure from the worksheet). The learner needs to identify whether the given representation indicates a pure substance or a mixture (of elements or compounds) along with the state (solid, liquid or gas). The link presents details about how to sequence the activities. These cards can be used by teachers to teach particle model of matter and the representations about pure substances/mixtures along with states of matter at the sub-micro level.
Definitions in chemistry
*https://edu.rsc.org/resources/definitions-in-chemistry/1088.article
This resource is about statements that are used to define terms such as elements, compounds, atoms and molecules. The learner is expected to reflect on the given statement and whether it appropriately defines the term (or not), along with justification. Thus, the relatively simple sheets help to critically look at the language used to define chemistry terms.
Chemical misconceptions – Elements, mixtures and compounds
*https://edu.rsc.org/download?ac=13301
This worksheet focuses on sub-micro representations of concepts. Learners need to identify the category to which the given representation belongs and explain their answer. It is important to elicit justification to understand learners’ thinking. Perhaps teachers could reflect upon the ways these representations are drawn before they use it in the classrooms.
Atoms, elements, molecules, compounds and mixtures
*https://edu.rsc.org/download?ac=17658
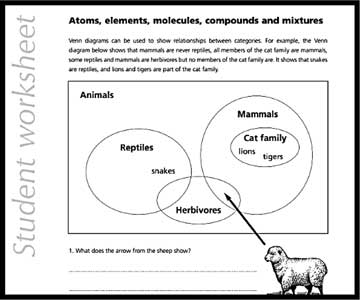
This worksheet introduces teachers to the Venn diagram, a pictorial depiction used to indicate logical relations among a finite set of categories. It is an extension activity that can be used only after the main topics are taught. It encourages teachers to develop similar worksheets for other topics in chemistry. This worksheet offers a good way to understand one of the simple tools that can be used to consolidate the interconnections among different pieces of knowledge.
Chemical misconceptions – Ionic bonding
*https://edu.rsc.org/download?ac=13324
This worksheet provides a representation for sodium chloride (NaCl), an ionic compound, along with 20 statements related to the diagram. Learners are expected to state whether the given statements are true or false. This worksheet can be used very effectively as a revision tool. It represents yet another way of developing effective instructional material. The worksheet also presents an answer key for the teachers’ reference.
Chemical bonding
*https://edu.rsc.org/resources/chemical-bonding/1140.article
This document is designed for teachers and it presents a discussion about the multiple conceptual frameworks used by learners to explain chemical bonding. The discussion highlights the misconceptions and the thinking that leads to such ideas. The resource would be engaging for any passionate teacher teaching chemistry as it presents a rich discussion about core topics of chemical bonding.
Beyond appearance
*https://edu.rsc.org/download?ac=15564
A freely downloadable book titled Beyond appearance: Students’ Misconceptions About Basic Chemical Ideas by Vanessa Kind, a Professor of Education, Durham University, UK is another important resource that deals with students’ misconceptions. It collates prominent misconceptions about a wide range of chemistry concepts from several CER studies. The book describes the evolution of the thought process among learners with progression in age. Most importantly, it presents discussions about some ways to overcome these misconceptions. The discussions in the book can even be used by teachers at higher secondary or undergraduate levels.
*All the links presented in the article were accessed in April 2020.
References
- Education | Royal Society of Chemistry. https://edu.rsc.org/ Accessed in April 2020.
- Johnstone, A. H. (1991). Why is science difficult to learn? Things are seldom what they seem. Journal of Computer Assisted Learning, 7(2), 75-83, accessed in April 2020.
- Kind, V., Beyond appearance: Students’ Misconceptions About Basic Chemical Ideas. Retrieved from https://edu.rsc.org, accessed in April 2020.
The authors are with the Homi Bhabha Centre for Science Education (HBCSE), Tata Institute of Fundamental Research (TIFR), Mumbai. They can be reached at savital@hbcse.tifr.res.in, hanza@hbcse.tifr.res.in.
