Vinay B R
During a training programme for 40 chemistry teachers, I was confronted with the following question: What is the role of a teacher? Is he/she a transmitter of knowledge? Or a person who facilitates knowledge construction? Or someone who builds a child’s personality and outlook? I am pretty sure this is neither an easy question to answer, nor does it have a unique answer.
Though the answer to this question transcends academics, I decided to attempt to answer it in the context of academics. It occurred to me that a quote (which I am very fond of) would do a fantastic job in answering the question in a concise and effective way. And so I quoted:
“A teacher is one who makes himself progressively unnecessary.” – Thomas Carruthers
However, much to my surprise, this quote did not succeed in communicating what I intended. Therefore, it was necessary to develop a context which these teachers could relate to. Since the attendees of the training programme were all chemistry teachers, I wondered: why not make use of a graph which is central to the understanding of any chemical reaction and which almost all chemistry teachers are aware of? Yes, it’s the “Free energy versus Course of Reaction” graph (figure 1).
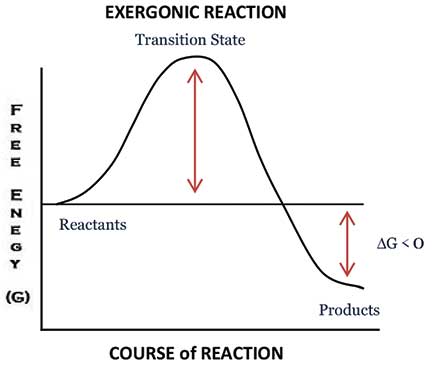
Though it’s not necessary to go into the intricate details of the graph, it’s important to note certain key features which come in handy to explain the role of a teacher. The key features of the graph are:
- An exergonic reaction is one where there is a net release of free energy to the surroundings. Since chemical reactions mainly consist of forming and/or breaking of chemical bonds, exergonic reactions release energy by breaking less stable chemical bonds and forming more stable bonds. Because the reactants lose energy, change in Gibbs free energy (ΔG) is negative under constant temperature and pressure. An example of an exergonic reaction is cellular respiration: C6H12O6 (glucose) + 6O2 –> 6CO2 + 6H2O + Energy. The released energy is used for cell activities.
- Though exergonic reactions are thermodynamically spontaneous in nature, they often require an input of energy to start the reaction, called the activation energy (EA). Once the required activation energy is provided by an outside source, the reaction proceeds and energy is released. This results in a net gain in energy in the surrounding system, and a net loss in energy from the reaction system; hence, the change in Gibbs free energy is negative (ΔG<0). A negative ΔG denotes that the reaction is spontaneous and thermodynamically favourable.
Now, we can use this graph to explain the role of a teacher in a child’s life by comparing and contrasting the key features of the graph to the key roles of a teacher.
- To begin with, one can assume children to be in the reactant stage before schooling and product stage after schooling. So the course of reaction on the x-axis of the above graph correlates to the course of schooling in a child’s life. Similar to how the products are more stable than reactants in an exergonic reaction, we ideally expect schooling to bring about that stability in the lives of children. Also, similar to how the energy released can be used to do useful work, one expects the products of schooling to be useful to the larger society.
- Having said the above, we still haven’t come to the role of a teacher. It is important to note that though exergonic reactions are thermodynamically spontaneous in nature, they often require activation energy (EA) without which the reactants fail to reach the product stage. Yes, in my opinion, the true role of a teacher is to provide the required activation energy for students to go from a less stable state prior to schooling to a more stable and useful state at the end of schooling. In this context, I would like to divide the role of a teacher into four main phases (Figure 2).
- The Motivator – One who motivates a child to learn.
- The Sustainer – One who sustains the motivation. Without sustenance, the child falls back to the state of reactants. This is exactly what happens in a chemical reaction too. Only those molecules which get the required activation energy make it to the product stage while others fall back to being reactants.
- The Challenger – One who challenges and gives the final push for the students to cross the activation energy barrier. This is when the transition state is crossed. A child has transited from the state of being dependent on teachers to being an independent learner.
- The Unnecessary Phase – Once the child crosses the activation energy barrier, it is an easy slide to reach the stage of products. Here, there is no requirement of a teacher. A teacher has done everything for a child to be an independent learner by now. Also, this is when the teacher has truly lived up to the spirit of the quote by Thomas Carruthers.
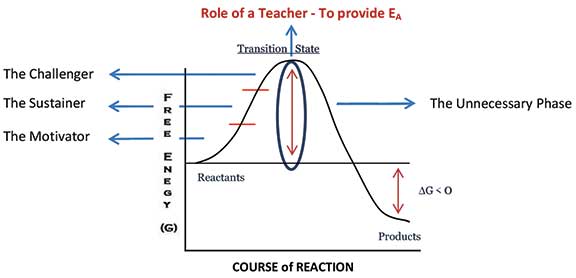
It’s not a hard and fast rule that every teacher should possess the ability to effectively function in all the four phases. But the teacher community as a whole should ensure that a child sufficiently gets exposed to all the phases and we should, until we become unnecessary to children.
Finally, it was successful. A chemistry context came in handy to explain the role of a teacher to chemistry teachers!
The author works as a research associate at Prayoga, a science education research organization based in Bengaluru. His research interest lies in the areas of synthetic organic chemistry and chemistry education. His work includes research-based design of instructional materials, tracking students’ learning progressions and their chemical reasoning in self-regulated learning environments. He is an avid trekker and a long-distance cyclist. He can be reached at vinayramesh.b@gmail.com.
