Yasmin Jayathirtha
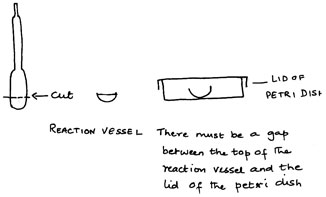 Among the non-metals, the halogens, chlorine, bromine and iodine, undergo a wide variety of reactions and are very important industrially. The elements and their compounds reflect periodicity very well and their displacement reactions exemplify change of reactivity down a group. Since they are so reactive, they are quite dangerous to handle. Chlorine and bromine, in particular, need to be in fume cupboards. Studying the chemistry of the halogens, indeed of any element, is a purely descriptive process and it will aid memory to have seen the reactions. Small Scale Chemistry allows students to observe reactions that they otherwise might only have to read about.
Among the non-metals, the halogens, chlorine, bromine and iodine, undergo a wide variety of reactions and are very important industrially. The elements and their compounds reflect periodicity very well and their displacement reactions exemplify change of reactivity down a group. Since they are so reactive, they are quite dangerous to handle. Chlorine and bromine, in particular, need to be in fume cupboards. Studying the chemistry of the halogens, indeed of any element, is a purely descriptive process and it will aid memory to have seen the reactions. Small Scale Chemistry allows students to observe reactions that they otherwise might only have to read about.
The preparation of the elements, chlorine, bromine and iodine should be a class demonstration. This will ensure that they are made in sufficient quantities, so that their colours can be seen. The other reactions of these elements can be studied at the tables, with pairs of students sharing a work surface and droppers of solutions.
The author works with Centre for Learning, Bangalore. She can be reached at yasmin.cfl@gmail.com.
