Aditi Chandrasekar
When an experimentalist studies the properties of a material, it is natural that the sample used is of a size visible to the eye and is easily handled. For example, a piece of zinc added to hydrochloric acid solution, a semiconductor chip used in an electrical study, etc. A student of science has a fairly good understanding about the various materials in their bulk state. However, can it be assumed that all properties remain unchanged when a very small sample of the same material is used? Common sense would suggest that the properties of a material would remain unchanged irrespective of the size of the sample used, but what actually takes place is often outside the realm of common sense.
When silver is in the finely divided state and the particles have diameters of the order of 100 nanometres (1 nm = 10-9 m), the colour of the suspension of silver particles in water is a light yellow shade. These are silver nanoparticles dispersed in water. When light is incident on a sheet of silver it reflects most of the light and hence it shines. When light is incident on a suspension of silver nanoparticles, they strongly absorb light of wavelength 390 – 420 nm (depending on the capping agent). This can be seen in an absorption spectrum of silver nanoparticles but it is not observed for larger particles of silver.
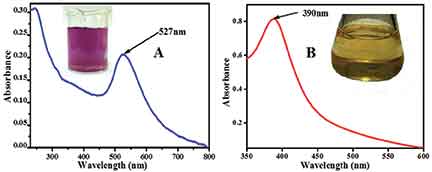
Figure 1 (A and B): Absorbance spectra of citrate capped gold nanoparticles and MSA (mercaptosuccinic acid) capped silver nanoparticles, respectively. The insets show the photographs of the corresponding nanoparticles.
The reason for this absorption feature is attributed to the presence of a large number of electrons on the outer surface of the nanoparticles. These surface electrons absorb light of a certain frequency and collectively oscillate creating a phenomenon known as surface plasmon resonance.
The reader may wonder how these nanoparticles are synthesised. A simple and widely used method is a synthesis in the solution phase. In this technique a salt of a metal ion (known as the Nanoparticle precursor) is stirred in water and then reduced by a common reducing agent like sodium borohydride. For example, the precursor for the synthesis of silver nanoparticles is silver nitrate and sodium borohydride is the reducing agent. The reaction may be represented as
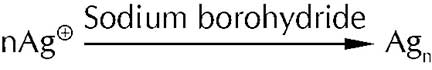
When the nanoparticles are formed they tend to aggregate and form a bulky mass due to their high surface area to volume ratio. In order to prevent aggregation and stabilize the nanoparticles a capping agent is added. This is generally a molecule containing an organic group which is sterically bulky or one with a long chain. These molecules added along with the precursor, surround the nanoparticles as they are forming and sterically hinder them from aggregating.
When metal clusters are even smaller than nanoparticles, their sizes are in the sub-nanometer regime and clusters such as these are known as quantum clusters: a new class of materials that are being studied over the past decade. Quantum clusters typically contain very few atoms, most often less than 100 atoms, and have discrete electronic energy levels, whereas larger collections of metal atoms have a continuous distribution of energy states. By virtue of the discreteness of energy levels, quantum clusters absorb and emit light showing luminescence. For example, silver quantum clusters made of 25 atoms of silver show red luminescence when irradiated with near ultraviolet or blur light. Luminescent quantum clusters find applications in sensors and imaging. Their low metal content makes them relatively less toxic and they may be used in drug delivery and tracking applications inside a living organism.
Traditionally, organic molecules that are fluorescent were widely used to stain and view biological cell structures. The disadvantage faced by the users of these dyes was that the fluorescence was not bright enough, and when the intensity of fluorescence of the excitation source is increased, molecules lose their ability to fluoresce on continued exposure to the excitation source. This is known as photobleaching. A different material in which both these disadvantages were overcome was then used. These are semiconductor nanoparticles capped with silica to render them water compatible. These are popularly known as quantum dots and are easily synthesised in a laboratory. Quantum dots have higher ability to absorb light than dyes and hence their fluorescence is around 10 – 20 times brighter than organic dyes. Their resistance to being photobleached is approximately 100 times more than dyes. When their size is below a certain radius, quantum dots of the same material show size dependent emission of light. As the size of the quantum dot decreases, the colour of the emission shifts to lower wavelengths. For example, larger quantum dots emit red light, smaller quantum dots of the same material emit green light and even smaller sized quantum dots emit blue light.

Figure 2: Quantum dots made of the same material and suspended in liquid show different coloured emissions depending on the particle size. As the size of the quantum dot increases the colour of the emitted light shifts from blue to red.
The explanation for this phenomenon is based on the “particle in a box” problem in quantum physics, where the spacing between two consecutive energy levels is inversely proportional to the width of the box. Here, the width of the box is analogous to the size of the quantum dot and the gap between the energy levels is analogous to the energy (or frequency) of the light photons emitted by the quantum dots upon excitation. The size dependent emission is a property that is not seen in large semiconductor particles.
A range of nanomaterials are being prepared and studied: thin films, nanorods, nanoflowers, nanoribbons, etc. Each of these structures has distinct characteristics that depend both on the material as well as their morphology. Nanoscience research has been growing rapidly and these fascinating structures have been finding successful applications in diverse fields like electronics, catalysis, biological, and water purification among others. The study of nanoscience is truly interdisciplinary, and in understanding it, the knowledge of all the four basic sciences is nurtured. Last but not least, a boundless imagination is ignited!
Even the little things make a difference!
The author is a chemistry teacher with an M.S in Chemical Sciences from the Indian Institute of Science Education and Research. Her research specialisation is in Nanoscience, which she worked on at the Indian Institute of Technology, Chennai. She can be reached at aditic2003@gmail.com.
