Yasmin Jayathirtha
The chemistry of carbon is first studied in the biology classes rather than in the chemistry ones, first as an introduction to ‘the chemicals of life’ and then during the study of respiration/photosynthesis. Of necessity, this is fairly sketchy and students (and teachers) are left with the idea that there are two chemistries of carbon, the inorganic and the organic.
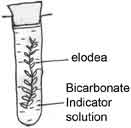 The idea of cycles of elements of course helps to dispel this notion that chemistry can be separated so neatly but it would be good to do experiments in the chemistry class that would highlight the role of carbon in biology and provide a link as to why it is studied in different compartments.
The idea of cycles of elements of course helps to dispel this notion that chemistry can be separated so neatly but it would be good to do experiments in the chemistry class that would highlight the role of carbon in biology and provide a link as to why it is studied in different compartments.
Carbon as the element is of course well known to all of us as graphite and diamond and is highlighted in the textbooks to demonstrate bonding and allotropy. It has long been my dream to burn a diamond and get carbon dioxide… but that experiment cannot be done.
Primo Levi, in his book The Periodic Table writes;
“So it happens, therefore, that every element says something to someone (something different to each) like the mountain valleys or beaches visited in youth. One must make an exception for carbon, because it says everything to everyone, that is, it is not specific in the same way that Adam is not specific as an ancestor – unless one discovers today (why not) the chemist-stylite who has dedicated his life to graphite or the diamond. And it is exactly to this carbon that I have an old debt contracted during what for me were decisive days. To carbon, the element of life my first literary dream was turned, insistently dreamed of in an hour and a place when my life was not worth much. Yes, I wanted to tell the story of an atom of carbon.”
The author works with Centre for Learning, Bengaluru. She can be reached at yasmin.cfl@gmail.com.
